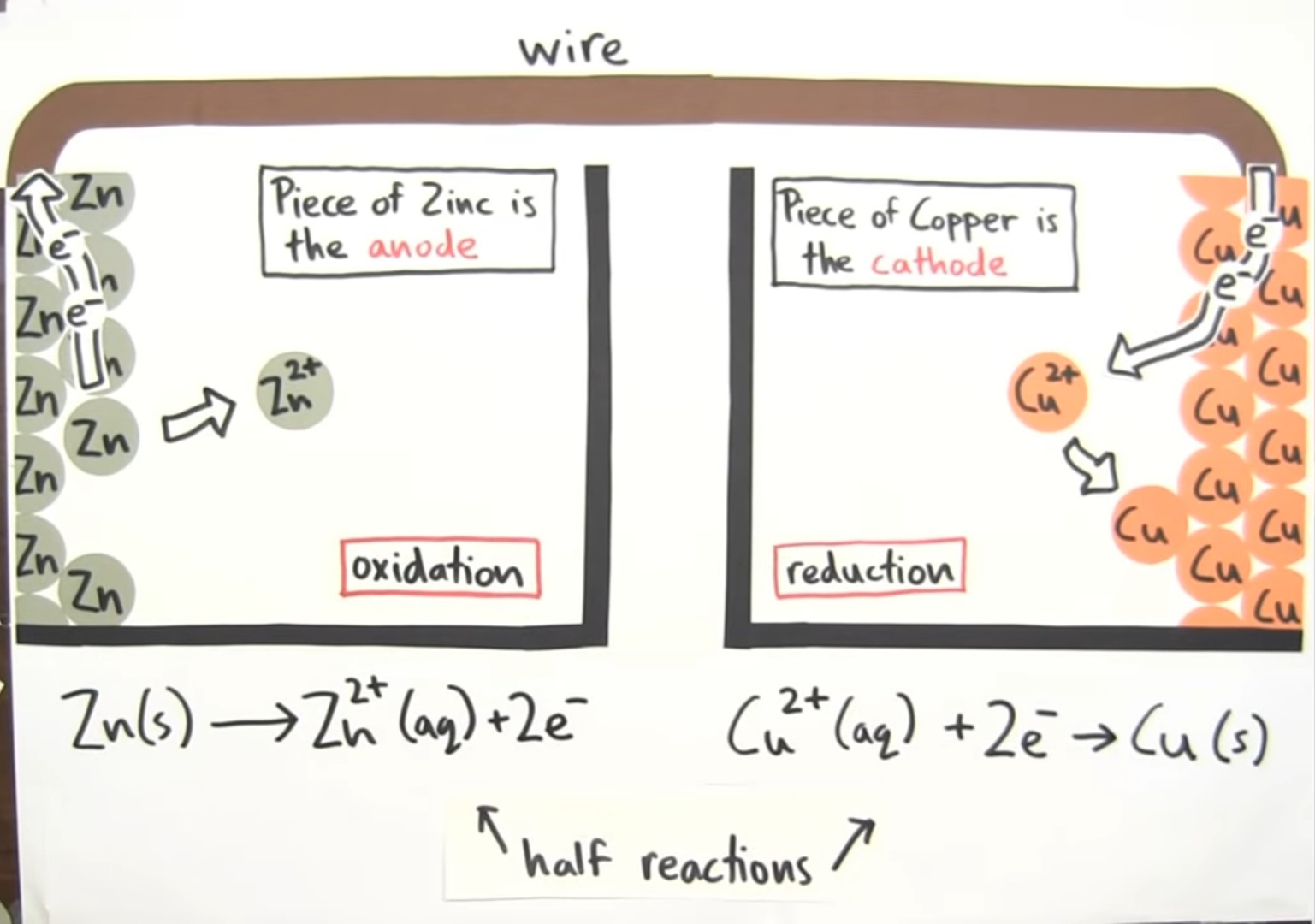
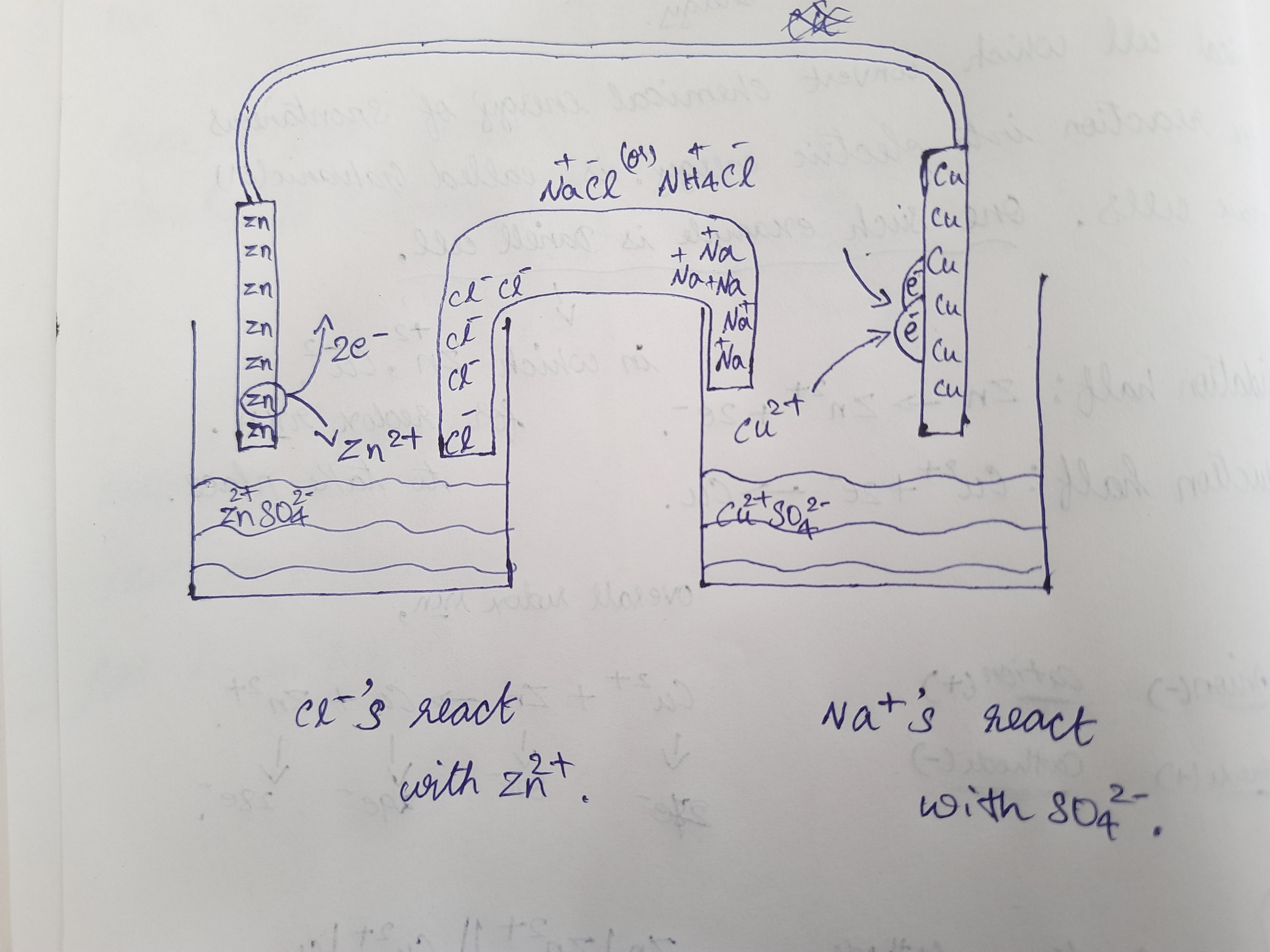
The working of a galvanic cell is quite simple. It involves a chemical reaction that makes the electric energy available as the end result. During a redox reaction, a galvanic cell utilizes the energy transfer between electrons to convert chemical energy into electric energy.
Galvanic cell utilizes the ability to separate the flow of electrons in the process of oxidization and reduction, causing a half reaction and connecting each with a wire so that a path can be formed for the flow of electrons through such wire. This flow of electrons is essentially called a current. Such current can be made to flow through a wire to complete a circuit and obtain its output in any device such as a television or a watch.
A galvanic cell can be made out of any two metals. These two metals can form the anode and the cathode if left in contact with each other. This combination allows the galvanic corrosion of that metal which is more anodic. A connecting circuit shall be required to allow this corrosion to take place